Abstract
Introduction: Acute myeloid leukemia (AML) with inv(16) is a common cytogenetic subtype found in 10-15% of children/young adults with AML. Inv(16) AML is one of the core binding factor (CBF) AMLs, characterized by the CBFB-MYH11 fusion gene generated by the chromosomal inversion [Bolouri, Nat Med 2018]. CBFB-MYH11 produces a fusion protein, CBFβ-SMMHC, which inhibits the CBF transcription factor complex via recruitment of transcriptional repressors to myeloid-promoting gene transcription sites. The other CBF AML, t(8;21) AML, results in the fusion protein RUNX1-CBFA2T1, which inhibits the CBF complex in a dominant negative fashion. Despite known similarities between t(8;21) and inv(16) AML, differing mutation profiles suggest distinct dependencies that may impact leukemia development and proliferation. Mutations in cohesin complex genes, which have essential functions in sister chromatid cohesion, higher order chromatin structure, transcriptional regulation, and DNA damage repair, are enriched in t(8;21) AML but do not occur in inv(16) AML, suggesting that cohesin complex function has different roles in the two CBF AML subtypes [Duployez, Blood 2016]. We hypothesized that inv(16) AML is dependent on intact cohesin, postulating that cohesin insufficiency may prevent AML development in an inv(16) murine model by altering chromatin accessibility of CBFβ-SMMHC and attendant changes in gene expression that impede leukemic transformation.
Methods: We crossed inducible Mx1-Cre+ CBFB56M mice with 2 separate cohesin models: mice with a heterozygous germline deletion of Smc3 (Smc3+/-) or mice with inducible deletion of one allele of Rad21 (Rad21+/fl). Following induction with 3 doses of polyinosinic:polycytidylic acid (pIpC) Mx1-Cre+ CBFB56M mice (CBFBΔ) develop AML [Kuo, Cancer Cell 2006]. We injected Mx1-Cre+ CBFB56M mice with and without cohesin gene deletions and CBFB+ controls with 3 doses of pIpC at 3-4 weeks of age and monitored for leukemia development for up to 1 year via monthly complete blood cell counts and peripheral blood immunophenotyping. We humanely euthanized moribund mice and harvested hematologic organs for further analyses.
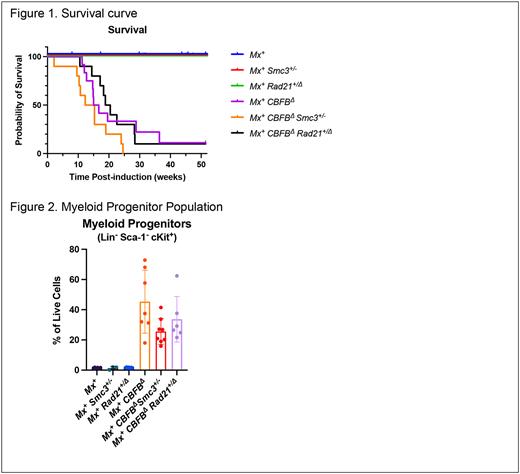
Results: Over 90% of Mx1-Cre+ CBFBΔ mice developed leukemia regardless of cohesin mutation status whereas no leukemia occurred in CBFB+ controls. Median survival was 15.9 weeks in Mx1-Cre+ CBFBΔ mice, 13.8 weeks in Mx1-Cre+ CBFBΔSmc3+/- mice, and 19.6 weeks in Mx1-Cre+ CBFBΔRad21+/Δ mice (Figure 1; p = 0.11). There was no difference in blood parameters between CBFBΔ groups and all mice with leukemia demonstrated splenomegaly with variable hepatomegaly. Leukemia immunophenotyping was similar between groups with loss of mature myeloid markers CD11b and Ly-6G/C, variable expression of CD71, and high expression of immature myeloid marker CD117. Both LSK cell (Lin-Sca1+CD117+) and myeloid progenitor (Lin-Sca1-CD117+) populations were increased in leukemic mice compared to controls. Mx1-Cre+ CBFBΔSmc3+/- mice had a significantly lower percentage of myeloid progenitors compared to Mx1-Cre+ CBFBΔ mice (Figure 2). Additional experiments including limiting dilution transplants and high-throughput sequencing are ongoing.
Conclusions: Haploinsufficiency of cohesin genes Smc3 and Rad21 do not prevent leukemia development in a mouse model of inv(16) AML. While loss of Smc3 reduced myeloid progenitors, loss of cohesin genes did not alter disease latency, clinical features, or immunophenotype of inv(16) AML. The reason for lack of cohesin mutations in patients with inv(16) AML is thus unclear. It is possible that the effects of cohesin mutations are redundant with CBFβ-SMMHC, thus there is no selective pressure for acquisition of a cohesin mutation in inv(16) AML. It is also plausible that experimental factors influenced our results. Mutation order can impact leukemogenesis, and it is possible the mutation order used in our study may not reflect the order of acquisition in patients. Finally, there may be other aspects of the chromosomal inversion independent of CBFβ-SMMHC expression that impact cohesin function in inv(16) AML that are not recapitulated in this study. In conclusion, our work suggests that intact cohesin function is not required for the development of inv(16) AML.
Disclosures
Rau:AbbVie Pharmaceuticals: Other: Spouse is employee and stock holder; Servier Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal